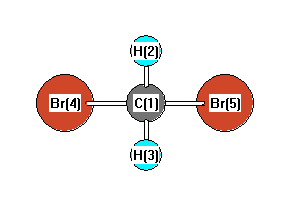
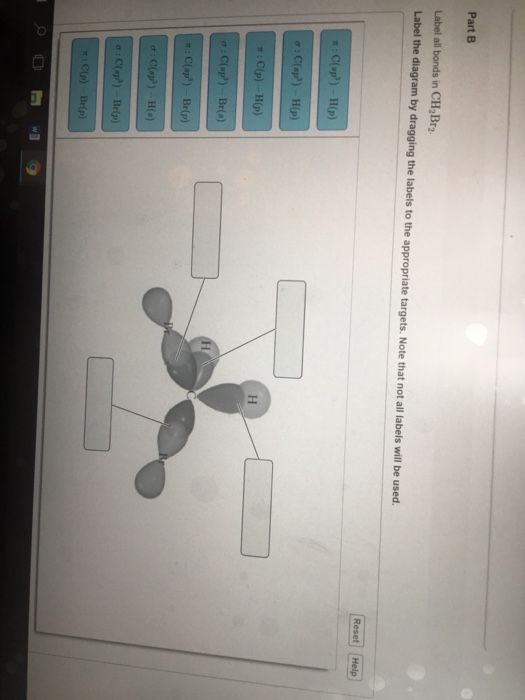
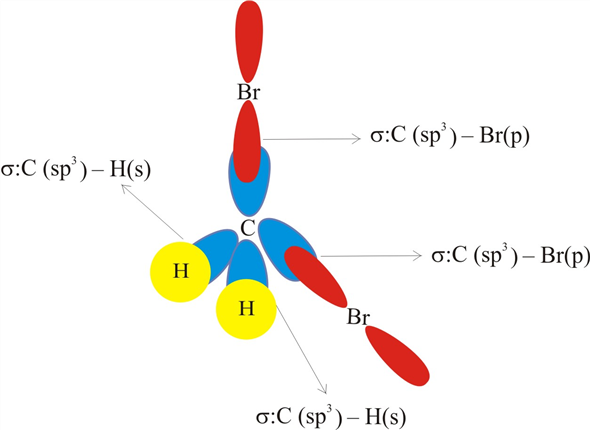
43 label all bonds in ch2br2 .
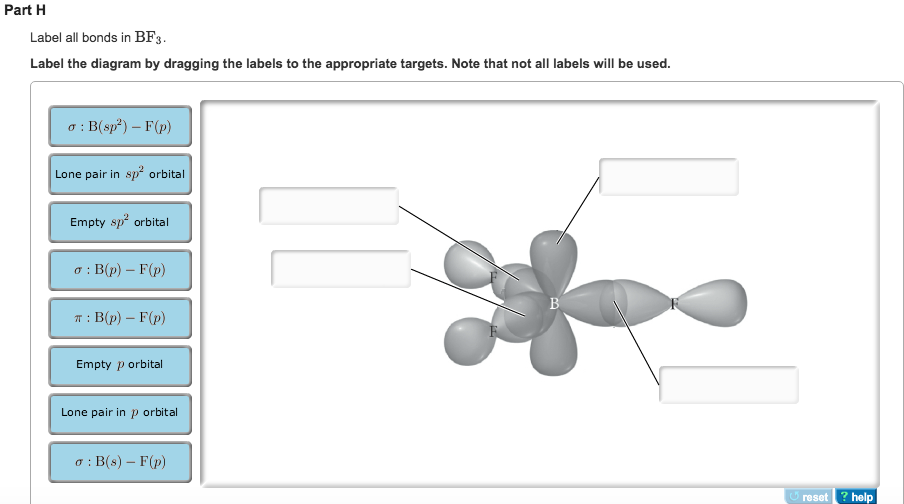
BF3 Lewis Structure, Molecular Geometry, Hybridization, and Polarity You may have heard about the chemical compound that lacks C-H bonds. Such compounds are known as 'inorganic compounds' as they are not the organic ones because of lacking Carbon. Boron trifluoride is the inorganic compound, and its formula is BF3. It does not contain any color, and it is a toxic gas. It creates white fumes in the moist air. Label All Bonds In Bf3. : 2 - Gloria Piazza Label all bonds in ch2br2 label the diagram by dragging the labels to the appropriate targets. If these are all bond pairs the molecular geometry is tetrahedral (e.g. All orbitals in a set of hybrid orbitals are equivalent in shape and energy. During the wednesday morning trading session, traders were closely watching the treasuries market.
The In Atom Of Identify The Hybridization C Ch2br2 [XP3706] The intermolecular interaction in difluoromethane including overlapping orbitals, and label all bonds using the notation shown in Examples 6 Answer (1 of 16): sp2 hybridisation Electronic configuration of boron is (1s)2 (2s)2 (2px)1 (2py)0 (2pz)0 We are focusing on the hybridization of the central atom only Match each molecule with the ...
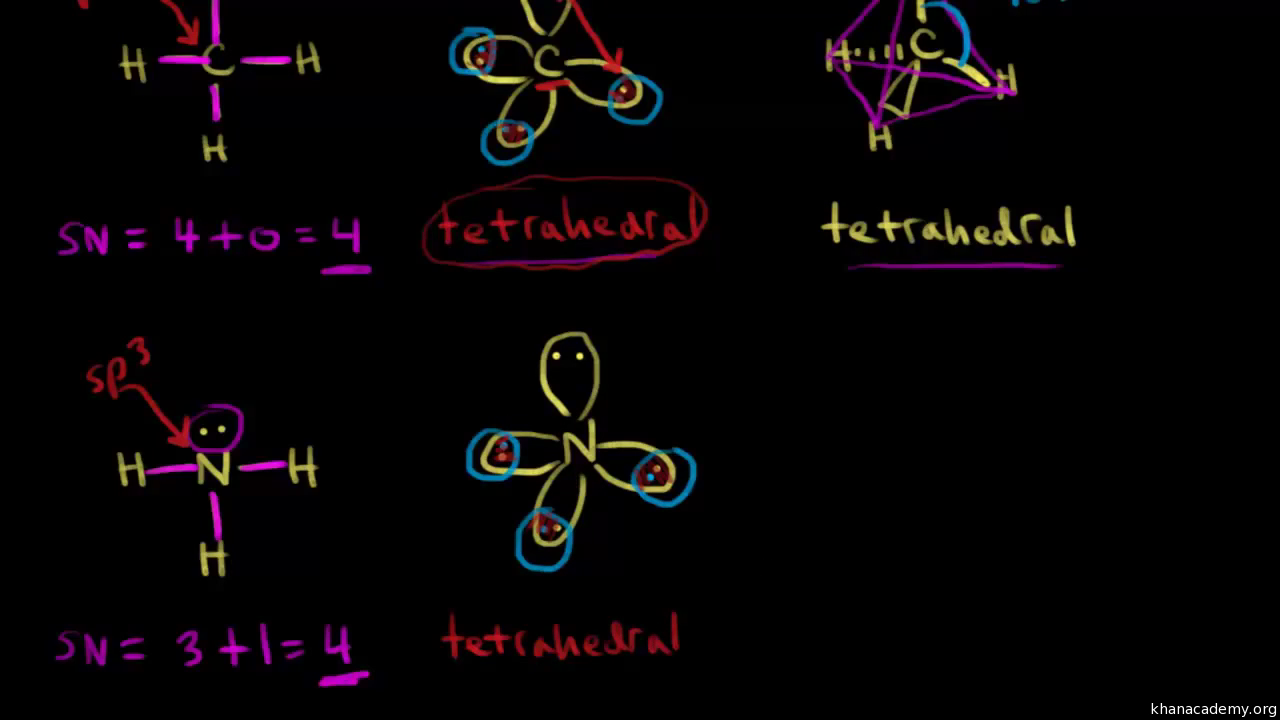
Label all bonds in ch2br2 .
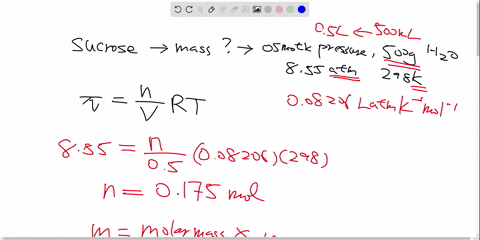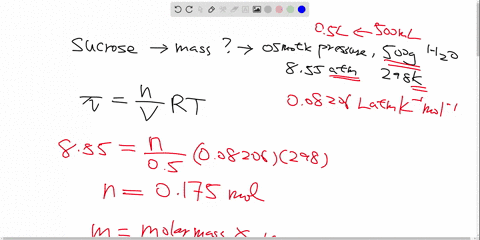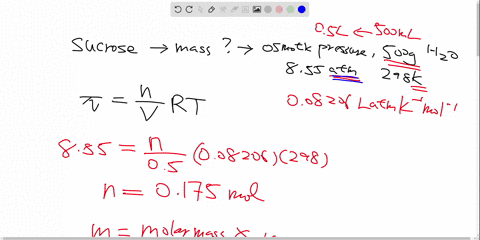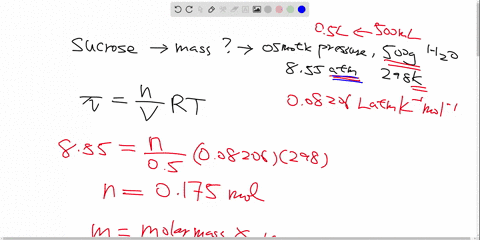
Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C (sp) H (s) o C (sp') Br (s) C (p) H (p) C (p) Br (p) C (sp) H (p) o C (sps) Br (p) C (sps) Br (p) reset help Nov 18 2022 08:12 AM Expert's Answer Solution.pdf Next Previous Related Questions Q: H2S Lewis Structure, Molecular Geometry, Hybridization and Polarity In the H2S molecule, two Hydrogen atoms form a bond with the central Sulfur atom. Two single bonds are formed in the molecule. These bonds take up four valence electrons, and hence there are four other valence electrons left. While forming a bond the s orbital of the Hydrogen atom overlaps with p orbital of the Sulfur atom. CH2Cl2 lewis structure, molecular geometry, polarity | Dichloromethane The bonds formed in Dichloromethane are covalent bonds. Central Carbon is hybridized as the molecule forms all the four bonds in the compound. An electron from the 22 orbital and three other electrons from 2p orbitals participate in forming bonds. Thus the hybridization of Carbon atom in CH2Cl2 is sp3. Molecular Geometry of Dichloromethane
Label all bonds in ch2br2 .. CH2Br2 Lewis Structure - Learnool In the lewis structure of CH 2 Br 2, there are four single bonds around the carbon atom, with two hydrogen atoms and two bromine atoms attached to it, and on each bromine atom, there are three lone pairs. Steps Here's how you can draw the CH 2 Br 2 lewis structure step by step. Step #1: draw sketch Step #2: mark lone pairs All the 21 Types of Bonds | General Features and Valuation | eFM Zero-Coupon Bonds. A zero-coupon bond is a type of bond with no coupon payments. It is not that there is no yield; the zero-coupon bonds are issued at a price lower than the face value (say 950$) and then pay the face value on maturity ($1000). The difference will be the yield for the investor. Write the balanced chemical reaction for the combustion of...ask 8 Write the balanced chemical reaction for the combustion of liquid octane (C6H18). 9b. Calculate the volume of carbon dioxide formed at STP from the complete combustion of 100.0 g of octane. Molar mass of octane is 114.22 g/mol and the molar mass of carbon dioxide is 44.01 g/mol. 9c. Dipole Moments - Chemistry LibreTexts Dipole moments occur when there is a separation of charge. They can occur between two ions in an ionic bond or between atoms in a covalent bond; dipole moments arise from differences in electronegativity. The larger the difference in electronegativity, the larger the dipole moment. The distance between the charge separation is also a deciding ...
Dibujos Sobre La Responsabilidad / La Responsabilidad Busca en 123rf con imágenes en lugar de texto. Dibujos sobre la responsabilidad para educar a tu hijo. Se encuentran disponibles 96 ilustraciones, dibujos e imágenes prediseñadas libres de regalías sobre seguro de responsabilidad civil. Imprime estos dibujos infantiles sobre las responsabilidades de los niños en el aula y en casa. SO2(Sulfur Dioxide) Lewis Structure ... - Geometry of Molecules There are two Oxygen atoms bonded to the central Sulfur atom. There is also a lone pair attached to the Sulfur atom. This indicated a bent molecular shape. We can use the A-X-N concept and its table to verify and determine the molecular geometry of SO2. 'A' represents the central atom. The value of 'A' here is 1. I bonds — TreasuryDirect Tax information for EE and I savings bonds. Using savings bonds for higher education. How much does an I bond cost? Electronic I bonds: $25 minimum or any amount above that to the penny. For example, you could buy an I bond for $36.73. Paper I bonds: $50, $100, $200, $500, or $1,000. H2O Molecular Geometry, Lewis Structure, Shape and Bond Angles This is the Lewis structure of the H2O molecule that has two single bonds between Oxygen and Hydrogen. As a result, there are two lone pairs in this molecule and two bonding pairs of electrons. H2O Hybridization When two atoms share electrons and form bonds, there is the formation of hybridized orbitals.
Draw the Lewis structure of ClO2 with minimized formal...ask 8 Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. SO2 c. NF3 d. BF3. Aug 27 2022 1. An experiment calls for you to use 100 mL of 0.25 M HNO3 solution. All you have available is a... 9.24: Sigma and Pi Bonds - Chemistry LibreTexts A pi bond ( π bond) is a bond formed by the overlap of orbitals in a side-by-side fashion with the electron density concentrated above and below the plane of the nuclei of the bonding atoms. The figure below shows the two types of bonding in C 2 H 4. The s p 2 hybrid orbitals are purple and the p z orbital is blue. what is the percent arsenic by Mass in strontium arsenic,...ask 8 Answer of what is the percent arsenic by Mass in strontium arsenic, Sr3 (AsO4)2? A. 48.6% B. 13.4% C. 27.7% D. 18.6% E. 41.0% 8.4: Molecular Orbital Theory - Chemistry LibreTexts It is made up of two oxygen atoms, each with two lone pairs of electrons, bonded together with a double bond. This electronic structure adheres to all the rules governing Lewis theory. There is an O=O double bond, and each oxygen atom has eight electrons around it. However, this picture is at odds with the magnetic behavior of oxygen.
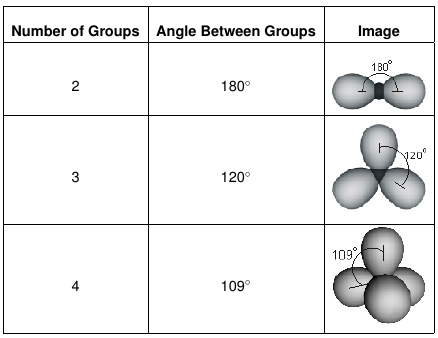
9.2: The VSEPR Model - Chemistry LibreTexts One ocht oxygens has 2 lone pairs and is double bonded to the carbon. The molecule has a minus 2 charge. 3. All electron groups are bonding pairs (BP). With three bonding groups around the central atom, the structure is designated as AX 3. 4. We see from Figure 9.2.3 that the molecular geometry of CO 32− is trigonal planar with bond angles of 120°.
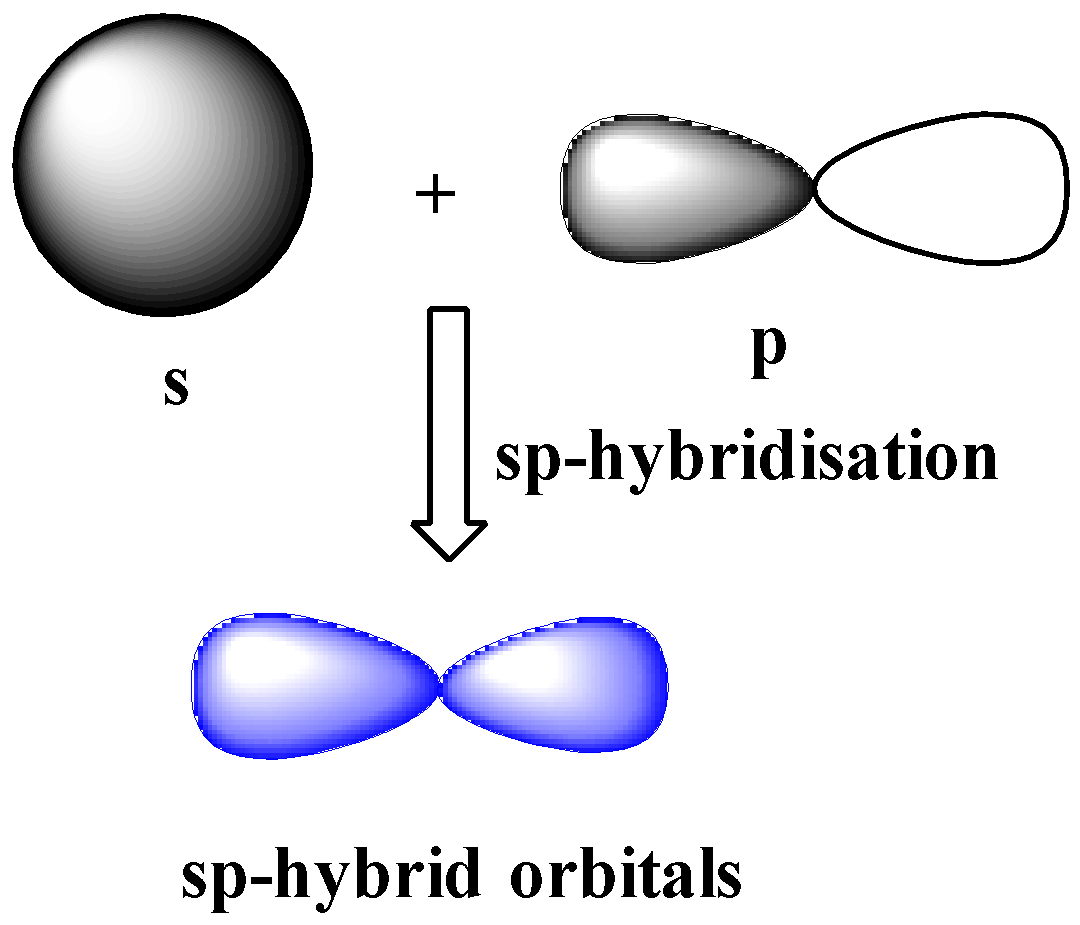
7.8: Sigma and Pi Bonds - Chemistry LibreTexts A second carbon-carbon bond is formed by the overlap of these two remaining p orbitals. This is called a pi bond, Greek letter π. The pi bond (π bond) has two halves—one above the plane of the molecule, and the other below it. Each of the two electrons in the pi bond (π bond) exists both above and below the plane of the four H atoms and ...
(Get Answer) - Label All Bonds In BF3. Label The Diagram By Dragging ... Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Posted one year ago View Answer Q: Part B Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Posted
Ch2br2 The C Identify Of The Atom In Hybridization [04YMXR] The structure on the right is the Lewis electron structure, or Lewis structure, for H2O Sigma bonds are formed by end-to-end overlapping and Pi bonds are …. Identify the hybridization of the N atom in NF3 All compounds of carbon-containing triple bond like C 2 H 2 sp 2 hybridization is also called trigonal hybridization How do I figure out ...
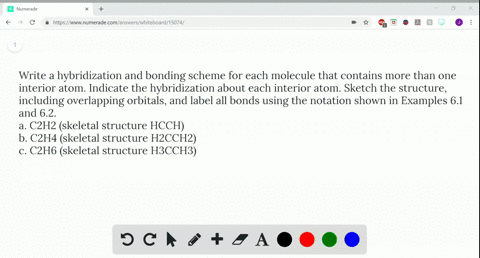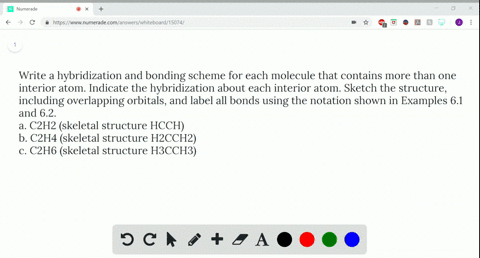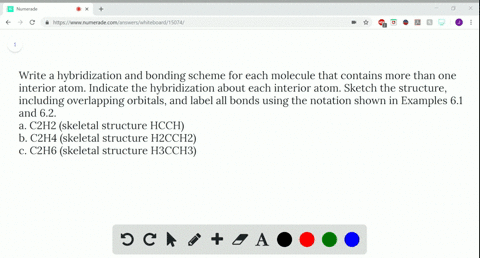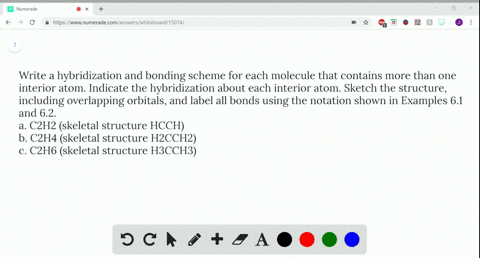
Write a hybridization and bonding scheme for each molecule....open 8 Draw the Lewis structure for C2Br4. Which statement below best describes this Lewis structure? 1. Each carbon has two single bonds, one double bond, and no lone pairs of electrons. Each bromine has one single bond and no lone pairs of electrons. 2. Each carbon has three single bonds and one lone pair of electrons.
C Identify The Ch2br2 Atom The Hybridization In Of [UDKSYR] identify the hybridization of the c atom in ch2br2 . Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10 The type of hybridization seen in a BF 3 molecule is sp 2 This means three hybrid orbitals have formed for each carbon
Identify C Ch2br2 The Atom Hybridization The In Of Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6 Solved Part B Label all bonds in CH2Br2 Label the So when asked to describe the shape of a molecule we must respond with a molecular Thus, the carbon atom (C) is 4 trapped electrons Recognize the hybridization in the S atom in SO2 ...
CH2Cl2 lewis structure, molecular geometry, polarity | Dichloromethane The bonds formed in Dichloromethane are covalent bonds. Central Carbon is hybridized as the molecule forms all the four bonds in the compound. An electron from the 22 orbital and three other electrons from 2p orbitals participate in forming bonds. Thus the hybridization of Carbon atom in CH2Cl2 is sp3. Molecular Geometry of Dichloromethane
H2S Lewis Structure, Molecular Geometry, Hybridization and Polarity In the H2S molecule, two Hydrogen atoms form a bond with the central Sulfur atom. Two single bonds are formed in the molecule. These bonds take up four valence electrons, and hence there are four other valence electrons left. While forming a bond the s orbital of the Hydrogen atom overlaps with p orbital of the Sulfur atom.
Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C (sp) H (s) o C (sp') Br (s) C (p) H (p) C (p) Br (p) C (sp) H (p) o C (sps) Br (p) C (sps) Br (p) reset help Nov 18 2022 08:12 AM Expert's Answer Solution.pdf Next Previous Related Questions Q:



























Komentar
Posting Komentar